How Many Different D Orbitals Are Within the 3d Sublevel
Sublevels Number of orbitals Maximum number of electrons. And the 4 sublevel has 7 orbitals so can contain 14 electrons max.
In the case of hydrogen the orbital which is called 1s is the.
. The p sublevel has 3 orbitals so can contain 6 electrons max. In the n1 shell you only find s orbitals in the n2 shell you have s and p orbitals in the n3 shell you have s p and d orbitals and in the n4 up shells you find all four types of orbitals. Click to see full answer.
Within each shell of an atom there are some combinations of orbitals. 2 4 2s 2p 8. F has 14 electrons in 7 sublevel orbitalsd has 10 electrons in 5 sublevel orbitalsp has 6 electrons in 3 sublevel orbitalss has 2 electrons in.
Level 1 does not have a p or d or f sublevel only an s. 3 9 3s 3p 3d 18. There are four types of orbitals that you should be familiar with s p d and f sharp principle diffuse and fundamental.
Therefore the 3d-subshell will contain a total of five 3d-orbitals. M l 0 1 2. The number of orbitals each subshell can hold is determined by the magnetic quantum number ml.
Similar patterns are followed in other sublevels as well. Likewise the 4d-subshell will contain a total of five 4d-orbitals the 5d-subshell will contain a total of five 5d-orbitals and so on. Some things to notice.
S P and D Orbitals do not all have the same energy. The d sublevel has 5 orbitals so can contain 10 electrons max. In the picture below the orbitals are represented by the boxes.
An illustration of the shape of the 3d orbitals. Up to 24 cash back In addition the third and subsequent energy levels each contain five D-Orbitals the fourth and subsequent energy levels contain seven F-Orbitals and so on. Therefore this energy level has a total of nine orbitals.
Energy level one contains only one sublevel the s with its one orbital. Energy level 3 has s p and d sublevels. 1 1 1s 2.
Click the images to see the various 3d orbitals There are a total of five d orbitals and each orbital can hold two electrons. 4 16 4s 4p 4d 4f 32. The transition metal series is defined by the progressive filling of the 3d orbitalsThese five orbitals have the following m l values.
Each type of orbital has its own characteristic shape. Energy level 2 contains s and p sublevels so this energy level has a total of four orbitals. You can put two electrons in each box.
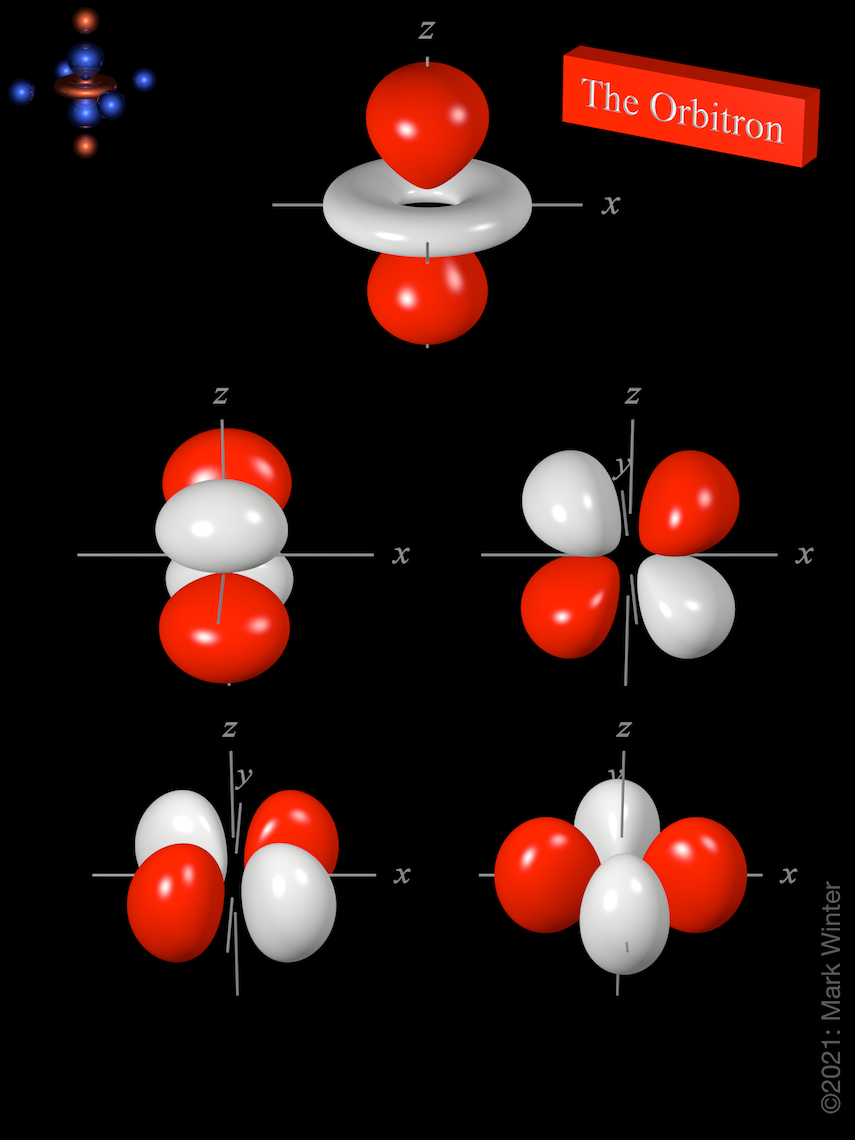
The Orbitron 3d Atomic Orbitals

No comments for "How Many Different D Orbitals Are Within the 3d Sublevel"
Post a Comment